Term: Tartaric acid
**1. History and Stereochemistry:**
– Known to winemakers for centuries
– Chemical extraction process developed in 1769 by Carl Wilhelm Scheele
– Important role in discovery of chemical chirality
– Property observed by Jean Baptiste Biot in 1832
– Louis Pasteur produced pure levotartaric acid in 1847
– Naturally occurring form is dextro tartaric acid
– Cheaper than enantiomer and meso isomer
– Modern textbooks refer to natural form as (2)-tartaric acid
– Dextro and levo form different crystal structures
– Anhydrous meso tartaric acid forms two polymorphs
**2. meso-Tartaric Acid:**
– Formed by heating dextro-tartaric acid in water
– Prepared from dibromosuccinic acid using silver hydroxide
– Separated from racemic acid by crystallization
– Used in Fehlings solution to bind to copper(II) ions
– Prepared in multistep reaction from maleic acid
**3. Reactivity and Derivatives:**
– L-(+)-tartaric acid reacts to produce dihydroxymaleic acid
– Dihydroxymaleic acid can be oxidized to tartronic acid
– Participates in several reactions
– Important derivatives include sodium ammonium tartrate and cream of tartar
– Tartar emetic is a resolving agent
– Diisopropyl tartrate used in asymmetric synthesis
– Median lethal dose is about 7.5 grams/kg for humans
– Used as antioxidant with E number E334 in foods
**4. Applications and Toxicity:**
– Used in pharmaceuticals for effervescent salts
– Combined with citric acid to improve taste of oral medications
– Diverse applications in pharmaceutical industry
– Tartaric acid toxicity in dogs can lead to fatal outcomes
– Studies suggest a connection between tartaric acid and toxic effects in canines
– April 2021 research highlights the potential dangers of tartaric acid for canines
**5. Tartaric Acid in Wine, Fruits, and Compound Summary:**
– Source of wine diamonds (potassium bitartrate crystals)
– Harmless but sometimes mistaken for broken glass
– Lowers pH of fermenting must and acts as preservative
– Provides tartness in wine
– Highest levels in grapes and tamarinds
– Also found in bananas, avocados, apples, cherries, citrus fruits, etc.
– Toxicity in Canines
– Compound Summary: Extensively studied in biochemical research, available on PubChem.
Tartaric acid is a white, crystalline organic acid that occurs naturally in many fruits, most notably in grapes but also in tamarinds, bananas, avocados, and citrus. Its salt, potassium bitartrate, commonly known as cream of tartar, develops naturally in the process of fermentation. It is commonly mixed with sodium bicarbonate and is sold as baking powder used as a leavening agent in food preparation. The acid itself is added to foods as an antioxidant E334 and to impart its distinctive sour taste. Naturally occurring tartaric acid is a useful raw material in organic chemical synthesis. Tartaric acid, an alpha-hydroxy-carboxylic acid, is diprotic and aldaric in acid characteristics and is a dihydroxyl derivative of succinic acid.

| |
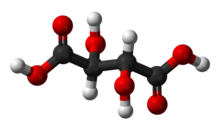 Ball-and-stick model of L-(+)-tartaric acid
| |
| Names | |
|---|---|
| Preferred IUPAC name
2,3-Dihydroxybutanedioic acid | |
| Other names
Tartaric acid
2,3-Dihydroxysuccinic acid Threaric acid Racemic acid Uvic acid Paratartaric acid Winestone | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.121.903 |
| E number | E334 (antioxidants, ...) |
| KEGG | |
| MeSH | tartaric+acid |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H6O6 (basic formula) HO2CCH(OH)CH(OH)CO2H (structural formula) | |
| Molar mass | 150.087 g/mol |
| Appearance | White powder |
| Density | 1.737 g/cm3 (R,R- and S,S-) 1.79 g/cm3 (racemate) 1.886 g/cm3 (meso) |
| Melting point | 169, 172 °C (R,R- and S,S-) 206 °C (racemate) 165-6 °C (meso) |
| |
| Acidity (pKa) | L(+) 25 °C : pKa1= 2.89, pKa2= 4.40 meso 25 °C: pKa1= 3.22, pKa2= 4.85 |
| Conjugate base | Bitartrate |
| −67.5·10−6 cm3/mol | |
| Hazards | |
| GHS labelling: | |

| |
| Danger | |
| H318 | |
| P280, P305+P351+P338+P310 | |
| Related compounds | |
Other cations
|
Monosodium tartrate Disodium tartrate Monopotassium tartrate Dipotassium tartrate |
Related carboxylic acids
|
Butyric acid Succinic acid Dimercaptosuccinic acid Malic acid Maleic acid Fumaric acid |
Related compounds
|
2,3-Butanediol Cichoric acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
