Term: Graphene
**Discovery and History of Graphene**:
– Graphene is an atomic-scale hexagonal lattice of carbon atoms, an allotrope of carbon with semimetal properties.
– The name ‘graphene’ is derived from graphite, and its unusual electronic characteristics were explored by P. R. Wallace in 1947.
– Efforts to make thin graphite films began in 1990, leading to patents for graphene production in the early 2000s.
– Graphene was rediscovered, isolated, and investigated at the University of Manchester in 2004, leading to Geim and Novoselov’s Nobel Prize in Physics in 2010.
– The global market for graphene was $9 million in 2012, and IUPAC recommends using the term ‘graphene’ for individual layers.
**Structure and Properties of Graphene**:
– Graphene is a single layer of carbon atoms arranged in a hexagonal lattice with a molecular bond length of 0.142 nanometers.
– Carbon orbitals form hybrid sp orbitals, resulting in sigma and pi bonds that contribute to graphene’s exceptional properties.
– Graphene exhibits remarkable electron mobility exceeding 15000 cm/Vs, with linear energy momentum dependence and unique charge transport concerns.
– It is transparent, has high tensile strength, and is the thinnest two-dimensional material globally, finding applications in various sectors like semiconductors and composites.
– Graphene displays a chiral, massless Dirac electron behavior and unique electrical and magnetic properties, making it ideal for spintronics and quantum Hall effect studies.
**Commercialization and Market Trends**:
– Companies and research labs have been developing commercial applications of graphene since the early 2000s.
– The National Graphene Institute was established in 2014 at the University of Manchester with significant funding.
– Commercial manufacturers like Applied Graphene Materials and Thomas Swan Limited are based in North East England, while Cambridge Nanosystems focuses on large-scale graphene powder production.
– The market for graphene was $9 million in 2012, with research expanding into various subfields to explore graphene’s exceptional properties.
– Graphene’s unique properties have led to its application in sectors like semiconductor, electronics, batteries, and composites.
**Graphene Band Structure and Properties**:
– Graphene’s band structure displays semimetallic character with unique energy-wave vector dependence near Dirac points.
– Electron mobility in graphene is remarkable, with values exceeding 15000 cm/Vs, nearly independent of temperature between 10K and 100K.
– Graphene exhibits discrete changes in electrical resistance in nanoribbons, quantum Hall effect anomalies, and unique charge transport concerns.
– It shows a minimum conductivity order of 4e^2/h, positive and negative photoconductivity, and remarkable thermal and electrical conductivity.
– The Casimir effect, Van der Waals force, and optical properties of graphene contribute to its diverse applications and scientific interest.
**Advanced Properties and Applications**:
– Graphene’s interaction with the electromagnetic field enhances the Casimir effect, influenced by boundary conditions on graphene surfaces.
– It exhibits high opacity for an atomic monolayer, with optical properties influenced by the fine-structure constant.
– First-principle calculations study graphene’s electronic and optical properties, including excitonic properties and spin transport.
– Graphene’s magnetic properties include large-amplitude ferromagnetism in low-defect nanomeshes and record magnetoresistance at room temperature.
– Thermal conductivity in graphene is a significant area of study, with potential applications in various fields.
Graphene (/ˈɡræfiːn/) is an allotrope of carbon consisting of a single layer of atoms arranged in a hexagonal lattice nanostructure. The name is derived from "graphite" and the suffix -ene, reflecting the fact that the graphite allotrope of carbon contains numerous double bonds.
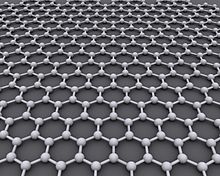
Each atom in a graphene sheet is connected to its three nearest neighbors by σ-bonds and a delocalised π-bond, which contributes to a valence band that extends over the whole sheet. This is the same type of bonding seen in carbon nanotubes and polycyclic aromatic hydrocarbons, and (partially) in fullerenes and glassy carbon. The valence band is touched by a conduction band, making graphene a semimetal with unusual electronic properties that are best described by theories for massless relativistic particles. Charge carriers in graphene show linear, rather than quadratic, dependence of energy on momentum, and field-effect transistors with graphene can be made that show bipolar conduction. Charge transport is ballistic over long distances; the material exhibits large quantum oscillations and large and nonlinear diamagnetism. Graphene conducts heat and electricity very efficiently along its plane. The material strongly absorbs light of all visible wavelengths, which accounts for the black color of graphite, yet a single graphene sheet is nearly transparent because of its extreme thinness. Microscopically, graphene is the strongest material ever measured.

Scientists theorized the potential existence and production of graphene for decades. It has likely been unknowingly produced in small quantities for centuries, through the use of pencils and other similar applications of graphite. It was possibly observed in electron microscopes in 1962, but studied only while supported on metal surfaces.
In 2004, the material was rediscovered, isolated and investigated at the University of Manchester, by Andre Geim and Konstantin Novoselov. In 2010, Geim and Novoselov were awarded the Nobel Prize in Physics for their "groundbreaking experiments regarding the two-dimensional material graphene". High-quality graphene proved to be surprisingly easy to isolate.
Graphene has become a valuable and useful nanomaterial due to its exceptionally high tensile strength, electrical conductivity, transparency, and being the thinnest two-dimensional material in the world. The global market for graphene was $9 million in 2012, with most of the demand from research and development in semiconductor, electronics, electric batteries, and composites.
The IUPAC (International Union for Pure and Applied Chemistry) recommends use of the name "graphite" for the three-dimensional material, and "graphene" only when the reactions, structural relations, or other properties of individual layers are discussed. A narrower definition, of "isolated or free-standing graphene" requires that the layer be sufficiently isolated from its environment, but would include layers suspended or transferred to silicon dioxide or silicon carbide.
